Favouritism and fake certifications mar India’s ventilator procurement
A tender by state-owned HLL Lifecare exposes favouritism and a lack of due diligence in the government’s attempts to address India’s ventilator shortfall

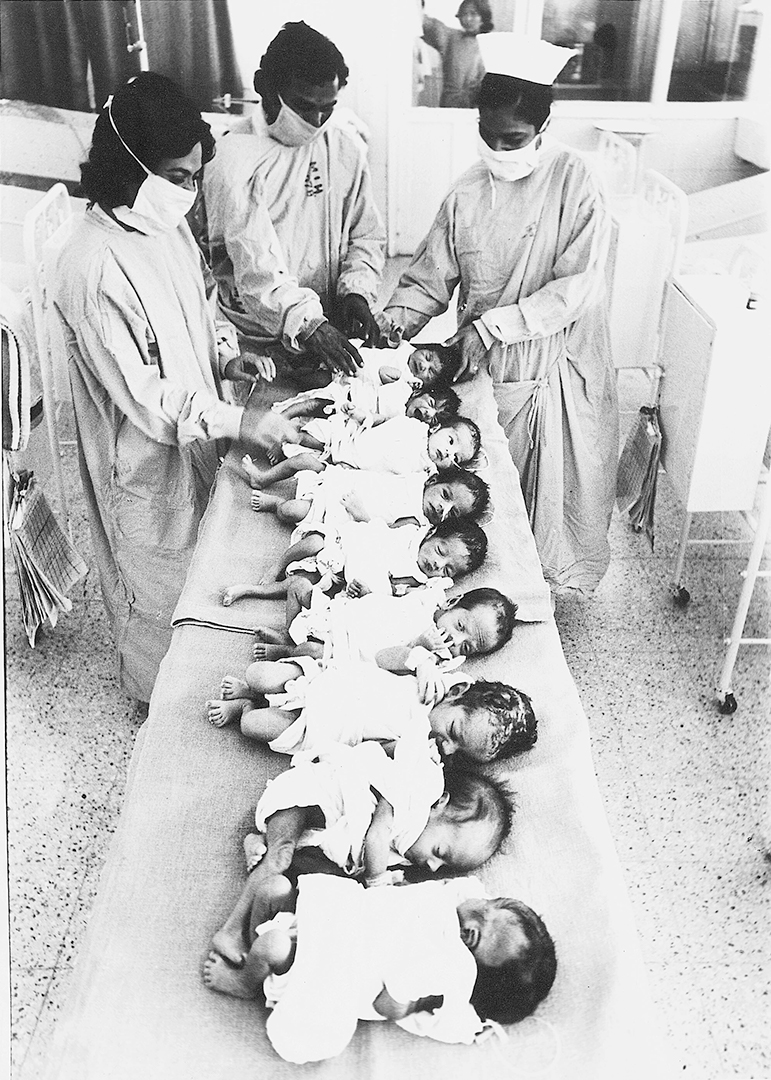
The number of Covid-19 cases in India has crossed the 40,000 mark. By the week’s end, it is expected to cross 50,000. Already, 1,389 people are reported to have died from the virus. Yet, even as India eases up on its five-week nationwide lockdown, the country is still desperately short of potentially life-saving equipment in the fight against the virus—ventilators.
According to projections from PD Vaghela, secretary of the department of pharmaceuticals, India requires at least 75,000 ventilators. Vaghela, who is leading a group tasked with procuring medical equipment for the government, says India has fewer than 20,000. To bridge this gap, the government has reportedly ordered upwards of 60,000 ventilators.
Noida-based AgVa is one of the firms that has been tapped for this. The startup is on its way to manufacturing half of the 20,000 ventilators that government-owned HLL Lifecare floated a tender for. With AgVa’s ventilators costing Rs 1.5-2 lakh ($2,000-2,600) per unit, the whole consignment was to be delivered by the end of April.
To date, AgVa—in partnership with automaker Maruti Suzuki—has only delivered 1,500 ventilators. Missed deadlines, though, are hardly the problem. No other Indian ventilator manufacturer has been able to deliver a single ventilator yet. Instead, the issue with AgVa’s ventilators stems from HLL’s tender itself.
Released on 27 March, one of the tender’s requirements was that all ventilators must be certified by the United States Food and Drug Administration (FDA).
AgVa’s ventilator is not FDA certified. Indeed, no Indian manufacturer has an FDA-certified ventilator. AgVa, though, has a certificate from a third-party company that says it is FDA compliant.
There are two problems with this. The FDA doesn’t certify companies, just products. And the FDA compliance can only be issued by the FDA itself.
In 2018, AgVa was certified by Unitas Certification Services, a company with a UK-based address. Unitas, incidentally, doesn’t appear to exist beyond its website. As recently as last month, AgVa received an IEC 60601 compliance certificate from NFI Certifications Ltd, another UK-registered entity, which appears to be a shell company. According to company filings, it has assets of £1 ($1.25). AgVa did not respond to questions sent via email.
Favouritism and fake certifications mar India’s ventilator procurement
Legitimate certification bodies in India are accredited by the National Accreditation Board for Certification Bodies (NABCB). Globally, accreditation is done by a member of the International Accreditation Forum (IAF), says Anil Jauhri, CEO of NABCB until July last year. “In most countries, in the absence of any law requiring certification bodies to register, accreditation is the only way of recognising a competent, authentic certification body.”
However, the absence of laws hasn’t just led to a rise in the importance of accreditation bodies. It has also spawned an entire industry of opportunistic and unscrupulous certification companies. “Such fake certifications have mushroomed. A difficulty has become a catastrophe,” says a senior executive at a medical equipment manufacturing company.
AgVa’s certification isn’t the only issue. There’s a fundamental problem with HLL’s tender. The open tender released by HLL is also based on the specifications of AgVa’s ventilator, according to the minutes of an HLL meeting obtained by The Ken. That on its own is not a problem. However, the specifications were released in the public domain a full 18 days after they were decided. So, while AgVa sat pretty, nailed on to win the tender, other manufacturers were at a disadvantage. HLL did not respond to questions sent by The Ken via email
Ventilators ain’t easy
Mysuru-based multinational healthcare company Skanray, which in collaboration with DRDO was to produce 30,000 ventilators, has delivered none. Automobile giant Mahindra & Mahindra, which announced with great fanfare that it would help plug the impending demand for ventilators, has gone silent.
Favouritism and fake certifications mar India’s ventilator procurement
Certify one certify all
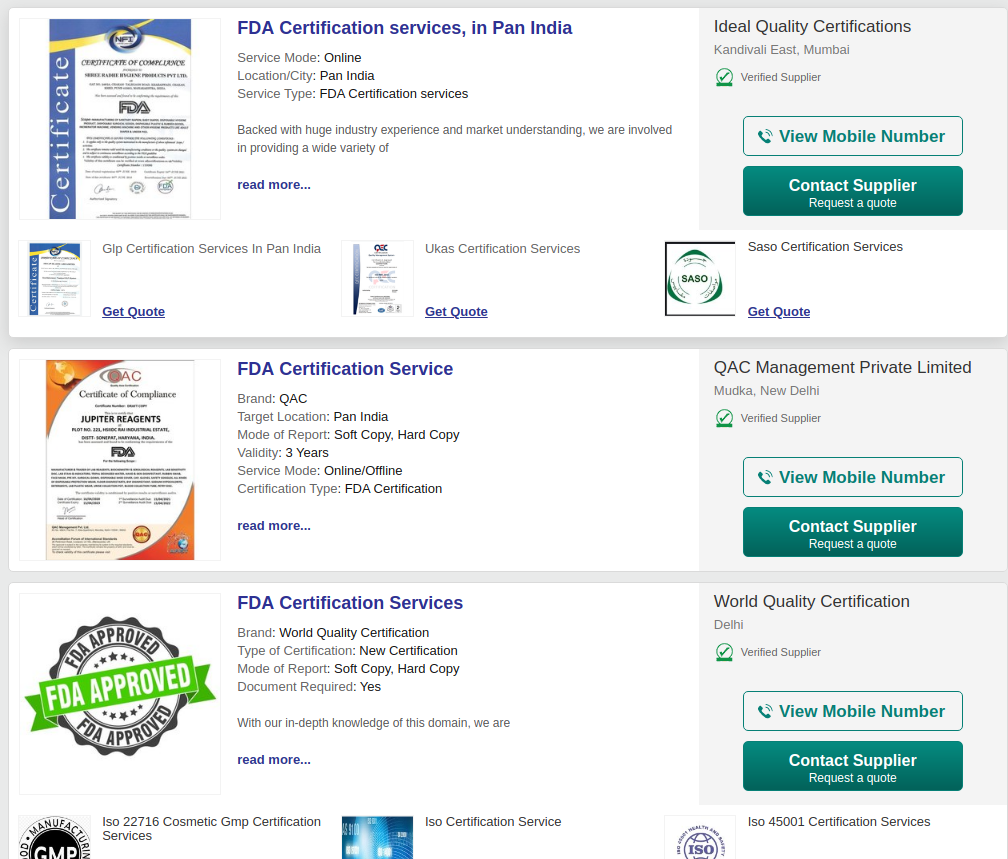
The modus operandi of a certification services company is straightforward. It usually has an Indian face, which openly markets itself, offering a variety of compliance certifications: FDA, CE marking, ISO, etc.
A second entity, supposedly overseas, issues the actual certificate. This adds an international flavour to proceedings, even as the issuing entity itself may not actually be incorporated. The price for this varies between Rs 10,000-30,000 ($132-$396) depending on your negotiation skills, with an “international” certificate promised within a week. There are few checks, if any, as to whether the product or company being certified is legitimate.
Through a serial number, this certification can be verified on the website of the international entity.
Interestingly, there is no ostensible link between the Indian entity and the overseas entity, which means multiple Indian certification services could use the same overseas entity.
Solutize Certification, a certification company based in Delhi, is a great example of this certification charade. It offers certifications in the name of Unitas Certification Services, which claims an address based in the United Kingdom (UK). According to Companies House, the UK’s registrar of companies, no such company is incorporated in the UK. Instead, Unitas appears to be merely a website and an address. The address itself leads to a residential property that is currently for sale.
The Ken has acquired certificates that Solutize issued to a number of companies, including AgVa. Electronics and electrical manufacturers Dynamic Systems and Hyglow Industries Limited, as well as industrial safety equipment manufacturer Balaji Industries also received certification from Solutize.

Solutize is hardly alone. Business e-commerce company IndiaMART shows at least 20 other companies that claim to offer FDA certification.
Mumbai-based Ideal Quality Certifications is another such company. The Ken acquired certificates issued in April 2020 to AgVa for its Covid ventilator as well as to small manufacturers such as Kevin Biotech Pvt Ltd and Baunetz Automotive Pvt Ltd to manufacture and sell personal protection equipment.
Certificates issued by Ideal Quality were in the name of UK-based NFI Certifications. Unlike Unitas, NFI is an actual company registered in the UK by an Indian citizen. The registered address, however, is a virtual PO box based in London. More than 47,000 companies are registered at the same address.
Favouritism and fake certifications mar India’s ventilator procurement
NFI Certifications goes further. Its website claims that it is accredited by the UK Akkreditering Forum Limited (UKAF). The UKAF, too, is registered by an Indian citizen and with a registered office address used by 19,000 other companies. Filings with Companies House show UKAF to be a dormant company with net assets of £1 ($1.24).
Unsurprisingly then, AgVa does not find a mention in the FDA’s list of approved ventilators. “It takes anything between a year to five to get FDA’s approval,” says an executive at a medical device manufacturing…
Favouritism and fake certifications mar India’s ventilator procurement
The FDA also prohibits the use of its logo in proposals or consulting deliverables, the way these certificates are used.
Tender troubles
A little over a month ago, The Ken reported on India’s lack of ventilators and how AgVa was stepping into the breach. The company built its own low-cost ventilator—from design to manufacturing—back in 2018. Priced at Rs 1.5 lakh ($2,000), far cheaper than most high-end ventilators, it seemed like an ideal candidate to help make up India’s ventilator shortfall. That it won HLL Lifecare’s tender, then, is unsurprising.
The manner in which it happened, though, raises questions of propriety. HLL’s tender process was riddled with arbitrary and seemingly whimsical changes, which, as it turns out, gave some companies a leg up on the competition.
On 27 March 2020, HLL Lifecare floated an open tender inviting manufacturers to supply an estimated 20,000 ventilators. The tender was an amendment to an earlier, larger tender to procure an assortment of essential Covid-related equipment, including PPE, masks, and sanitisers.
The following day, HLL released another amendment to the tender, relaxing some of the previous specifications. A few days later, on 31 March, it added six more specifications. It also decided on a further three specifications that same day, according to minutes of a meeting accessed by The Ken. These, however, were announced over two weeks later on 18 April. Curiously, these additional specifications were taken as is from AgVa’s ventilator:

While this delay in releasing specifications made no difference to AgVa’s bid, it handicapped the company’s competition, who were blindsided.
This wasn’t the only instance of ad hocism in the process. A healthcare device manufacturer working to build ventilators on a separate tender shared with The Ken the specifications given to them as an annexure dated 2 April. These specifications included those only released publicly on 18 April.
“It just can’t be done, period,” says an employee at a government-run institute that frequently floats tenders to procure expensive machinery used for research. “You can explain the same things in generic terms,” he says. “If it makes it very specific for one company, that tender itself is illegal.”
Due diligence
This chain of events exposes the sheer inadequacy of the government’s processes in the fight against the pandemic. Due diligence was lacking throughout. Not just in verifying certifications or updating the tender in a timely manner, but in the constitution of the tender itself.
The first tender, released on 27 March, mandated FDA approval or CE marking. No Indian ventilator has the former, while only Skanray—which exports to Europe—has the latter. Besides, there’s no singular list to check which products are CE certified. This was later amended to “reputed national/international certifying agency”. Apart from being incredibly vague, this opens the door to shady certification companies.
The need for certification itself flies in the face of the government’s earlier communication, which asked companies to come forward and build ventilators without needing to apply for permissions. India’s bureaucracy, it seems, isn’t even consistent with its own diktats.
Favouritism and fake certifications mar India’s ventilator procurement
“I think all the bodies are trying to do their best and are limited by the legacy of people, systems, and the processes they have, which they are also trying to improve,” says the senior executive quoted earlier.
Whichever way you slice it, though, India’s approach to certification is in desperate need of an overhaul. NABCB’s Jauhri acknowledges that even government entities are unaware of the nuances behind certification. “The MHA released guidelines for PPE which said that they must be certified by a reputed international/national agency. What is a reputed international agency? Is an agency based in Pakistan a reputed international agency?”
In the short term, Jauhri thinks that education is the only way out. “Educate, educate, educate,” he says. “There is no law. Legally there is no bar [to incorporate a certification company].” In the longer term, he believes regulation is the only way out. “Everyone must be regulated. Certification bodies, inspection bodies, labs, even accreditation bodies,” he says. “If there is no law, there is mayhem in the market.”